 |
Issue #5 - published by the REACTION project - August, 2013 |
 |
 |
 |
 |
 |
Launch of the primary care pilot |
|
 |
The REACTION primary care pilot began in the first quarter of 2013 at Chorleywood Health Centre offering 203 diabetes patients access to a range of REACTION health services.
Measure your blood glucose, blood pressure and weight at home, watch the results online and share them with your doctor and nurse who can give advice on your daily glucose, diet, activity and medication. Access your personal diabetes care plan and educational content to get to know more about your condition and to help you manage your diabetes better.
At Chorleywood Health Centre, this becomes reality for diabetes patients deciding to participate in the REACTION primary care pilot. As part of their usual diabetes care, they are offered a suite of services providing home monitoring of blood glucose, blood pressure, physical activity and diet.
"The aim is to achieve comprehensive protection against diabetic complications and co-morbidities and to promote pro-active disease management", explains Joanna Fursse, Research Project Coordinator at Chorleywood Health Centre.
"We will also be able to demonstrate how patients manage their medication and how they respond to education and suggestions for self-management of their health and use of services", she adds.
Whereas the patient can monitor own data through a patient portal, the primary care team manages the incoming patient data via a clinical portal which works as a tool for decision making.
"The REACTION services will enable online access to the patient’s monitoring data, clinical test results and personal diabetes care plan and give us additional information on daily glucose, blood pressure, self-reported diet, activity and medication compliance for our routine annual and 6 monthly diabetes reviews. We will also use the gathered data, together with usual review data and historic Electronic Patient Record data, to undertake risk stratification, thus identifying and providing appropriate intervention for those at highest risk", says Joanna Fursse.
Patients will have access to the patient portal for a minimum of two weeks twice per year. Enhanced versions of the primary care clinical portal and patient portal which add increased functionality and improvements in usability are being deployed during the summer of 2013.
/lbr/
to
the top  |
 |
 |
 |
 |
| |
Improving glycaemic management in general hospital wards |
|
 |
Based on current diabetes research, glucose management guidelines and extensive workflow analysis, REACTION partners have developed a decision support system for clinical professionals with the aim of improving hospital workflow and optimise insulin therapy.
"Research shows that diabetes is a common co-morbid condition in hospitalised patients. Poor glycaemic control is associated with an increased risk of infection, disability after hospital discharge and death and if diabetes is present, hospital stays are 50 % longer", explains Katharina Neubauer MSc., Medical University of Graz.
"To achieve good glycaemic control, the guidelines and general recommendations consider the implementation of a standardised subcutaneous insulin basal-bolus therapy as key intervention and request the development of integrated decision support strategies and systems to guide medical staff in the glucose management process", she continues.
In the development of GlucoTab, focus has therefore been on creating a decision support system which includes an algorithm for basal-bolus insulin therapy for better glycaemic control and which integrates with existing hospital systems and workflows.
Four steps to efficient glycaemic management
With the general wards at the Medical University of Graz as reference points, the decision support system has been developed in a stepwise approach which has included analysing glycaemic management workflow, developing and testing algorithms and investigating the safety, usability and efficacy of the support system.
Katharina Neubauer explains the four steps of the development process: 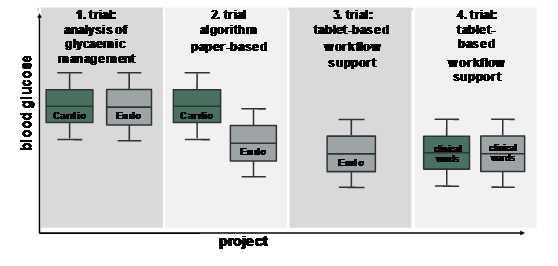
1. Assessment of the quality of physician-managed glycaemic control
Research points to glucose data collection, analysis and evidence based practice as the foundation of all glycaemic management efforts. The most recent guidelines recommend a pre-meal blood glucose (BG) target of less than 140 mg/dL and a random BG of less than 180 mg/dL for the majority of hospitalised patients with non-critical illness. As a first step, we assessed retrospectively the quality of physician-based glycaemic management in 50 patients at two general wards, considering the most recent recommendations for glycaemic control for non-critically ill patients (<140 mg/dL for pre-meal glucose). Glucose values were clearly above the recommended target (mean blood glucose levels: endocrinology: 175 ± 62 mg/dL; cardiology: 186 ± 68 mg/dL). Comparing the first half to the second half of the hospital stay showed neither a difference in glucose levels nor insulin dose despite frequent blood glucose measurements which demonstrates considerable glycaemic management effort but poor implementation into adequate insulin therapy. Further analysis by questionnaires indicated a lack of clearly defined blood glucose targets. Implementation of corrective measures, such as structured treatment protocols, is therefore regarded as essential tools to improve this situation.
Related paper: Failure to Control Hyperglycemia in NonCritically Ill Diabetes Patients
2. Develop a workflow integrated algorithm to establish glycaemic control and implement it in an electronic decision support system
An essential step to improving glycaemic management is to develop and test algorithms. Thus, the aim of our second trial was to evaluate glycaemic control and usability of a paper-based workflow-integrated algorithm for basal-bolus insulin therapy in a proof-of-concept study to develop a decision support system for management of hospitalised patients with type 2 diabetes. Average blood glucose levels in the algorithm group had a significantly higher percentage of glucose levels in the recommended target range compared to the standard group. Physicians’ adherence to the algorithm-calculated total daily insulin dose as well as nurses’ adherence to injecting the algorithm-calculated basal and bolus insulin doses were very high, which indicates that the workflow-integrated algorithm for basal-bolus therapy was efficacious in establishing glycaemic control and was well accepted by the medical staff.
Related paper: Efficacy, usability and sequence of operations of a workflow-integrated algorithm for basal-bolus insulin therapy in hospitalized type 2 diabetes patients
The findings of the second clinical trial support the implementation of the algorithm in an electronic decision support system: The GlucoTab system was developed by REACTION partners Medical University of Graz, Joanneum Research - Institute for Biomedicine and Health Sciences, Foundation for Research and Technology – Hellas and Fraunhofer SIT.
 It is a software system for improving the workflow of clinical professionals (nurses, doctors) when managing patients with diabetes type 2 in general wards. In addition, the software supports clinical professionals in determining the right insulin dose for patients with diabetes type 2. Medical data (blood glucose, creatinine), physiological data (age, weight) and demographic data (age) entered manually by clinical professionals or received automatically from the hospital information system are used in accordance with the clinical protocol to calculate the insulin dose. Visualisation of BG values and medication is provided and the workflow is supported by automatically displaying outstanding tasks. It is a software system for improving the workflow of clinical professionals (nurses, doctors) when managing patients with diabetes type 2 in general wards. In addition, the software supports clinical professionals in determining the right insulin dose for patients with diabetes type 2. Medical data (blood glucose, creatinine), physiological data (age, weight) and demographic data (age) entered manually by clinical professionals or received automatically from the hospital information system are used in accordance with the clinical protocol to calculate the insulin dose. Visualisation of BG values and medication is provided and the workflow is supported by automatically displaying outstanding tasks.
3. Investigate the safety, usability and efficacy of the GlucoTab
The aim of the third trial was therefore to investigate the safety, usability and efficacy of the GlucoTab system. The results of the clinical pilot trial indicate that 98.4 % of documentation of the blood glucose measurement, 98.8 % of basal insulin administrations, and 93.6 % of bolus insulin administrations were successfully performed with the GlucoTab system. Mean blood glucose was 144 mg/dL with improved treatment protocol of the study. 98.6 % of the suggested new total insulin dosage has been accepted by the physicians. 95.7 % of the basal insulin suggestions and 96.2 % of the bolus insulin suggestions have been accepted by the users. In summary, the GlucoTab system was efficient, provided good usability and was safe for patients.
4. Prove the usability of GlucoTab outside specialised wards
After successful testing of the GlucoTab system at the medical ward of Endocrinology, another trial at other clinical wards is currently running to reduce any potential bias from experienced nurses and to prove its usability to support in-hospital glycaemic management outside specialised wards.
All data and findings will be used for the certification process to gain a CE label which is mandatory for using the GlucoTab system in routine care. As a final step, a multicentre trial is planned for evaluating the impact of the GlucoTab system on intermediary outcomes (hyperglycaemia and hypoglycaemia), clinical outcomes (in-hospital mortality, hospital-acquired infections) and economic outcomes (length of hospital stay, hospital admission costs, re-admission) as recommended by the PRIDE consortium.
/lbr/
to
the top  |  |
 |
 |
 |
| |
In-hospital authorisation and access control in REACTION |
|
 |
Ensuring identification, authentication, authorisation and access control is one of the major tasks in terms of security within REACTION.
With the in-hospital application of the REACTION platform, medical personnel will use a mobile device to access patient information. As in the physical world, it is necessary to ensure that electronic patient records are only accessed by authorised medical staff such as nurses or physicians.
Once a staff member is registered on the REACTION platform, he or she is provided with a digital identity. Technically, this identity is based on a cryptographic key, though to the user it is just a user name and a password. In addition, certain access permissions are given to this identity. The permissions reflect the hospital’s security policy and restrictions as to which user can access what information. For example, a nurse may be authorised to read but not to modify a patient’s health record while a physician is allowed do both.
When a staff member requests access to patient information, he or she provides his/her identity and, by a cryptographic protocol, proves that he/she is the rightful owner of the claimed identity. For the in-hospital application, this authentication process is based on two factors: The user’s identity and the possession of a tablet device registered with and approved by the hospital’s IT staff. After successful authentication, the platform’s access control component enforces the permissions assigned to the user’s identity by either granting or denying access to the requested information.
The concept of roles
Managing each user’s permissions individually can become a burden. Therefore, REACTION’s access control uses the concept of roles. Roles are essentially sets of permissions that are uniformly used by a certain group, e.g., nurses, physicians, etc. Using roles, it is no longer necessary to assign individual permissions to new staff members. Instead, upon registration a new staff member is assigned one or more roles according to his or her job functions. These roles, in turn, automatically provide her with all the access permissions he/she requires.
Dynamic constraints
In certain situations, the standard role-based approach alone might not be flexible enough. For instance, a general security policy could define that each staff member may only access data of patients from his/her own ward. However, “own ward” depends on the user and is independent of any role. One approach to this would be to invent artificial roles, say, “cardiology nurse” and “endocrinology nurse”, even though both have the same permissions in their respective ward. REACTION uses another approach that does not cause an inflation of artificial roles and additional maintenance work. This approach is based on so called dynamic constraints, which can take into account arbitrary run-time information for access control decisions, e.g., a user’s identity and “his/her ward”. Simply put, a dynamic constraint is just a programmable condition that is evaluated during access in conjunction with rules from the access control policy. For instance, if Nurse Alice is requesting data of an endocrinology patient, her role as “nurse” would permit read access to patient information, in general, but an additional dynamic constraint, connected to the personnel database and attached to endocrinology data, would only agree if the requesting user, Nurse Alice, is found in the personnel database to be working in endocrinology. This example illustrates that using even a small and easily manageable set of roles, one can have very tight access controls on sensitive data.
/lbr/
to
the top  |
 |
 |
 |
 |
| |
Modelling blood glucose control |
|
 |
A central element in diabetes research is working with mathematical models of the glucose insulin metabolism as the basis for modern glucose control approaches.
"Offering automatic blood glucose control to patients with diabetes, including closed loop delivery of insulin, is a key goal in diabetes management. As a step towards this goal, mathematical models are used to provide valuable insight into the mechanisms of the glucose insulin metabolism and, coupled with glucose control algorithms, to offer solutions for automatic glucose control", says Stephan Schaller, Biological Modeling Expert and Control Engineer at Bayer Technology Services.
In REACTION, models of the glucose insulin metabolism have been developed, both for people with diabetes and for people without, to create closed loop control algorithms. The approach combines a generic, whole-body model of the glucose-insulin-glucagon regulatory system with a model for automatic glucose control (Figures 1 and 2) to encompass the complexity of the glucose-insulin-glucagon regulatory system and the mechanisms involved in hormonal glycaemic control. 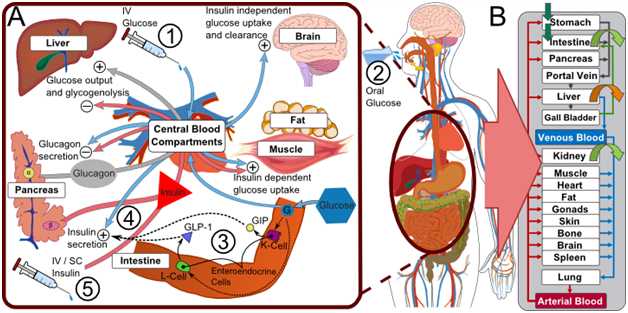 Figure 1 © Nature Publishing Group: The physiologically-based, whole-body model of the glucose-insulin-glucagon regulatory system couples three models for glucose, insulin, and glucagon. Section A gives an overview of pharmacodynamic interactions (what the injection of glucose/insulin does to the body). Section B shows the organ-level structure of the model. Source: A Generic Integrated Physiologically based Whole-body Model of the Glucose-Insulin-Glucagon Regulatory System.
Detailed modelling approaches offer more accuracy
Mathematical models of the glucose insulin metabolism are developed for many application areas to analyse the underlying mechanisms in individuals with and without diabetes, i.e. for target identification, risk analysis, clinical trial design, decision support and as model kernels for automatic blood glucose control of people with type 1 diabetes. Apart from developing closed loop control algorithms, models in REACTION have also been used to create frameworks for short-term risk assessment and to generate new knowledge through data analysis.
Over the years, the availability of highly informative but complex physiological data has steadily increased, paving the way for detailed modelling approaches which can provide more accuracy and prediction power for glucose control.
"The physiological model developed in REACTION provides a high level of detail and can account for multi-scale data such as cellular data, organ data, organism data as well as population data. It provides the necessary structural detail for the integration of these multi-scale data as well as detailed mathematical descriptions of physiologic processes and properties. Coupled with an accurate prediction model of the core dynamics of the blood glucose levels, adapted over time from continuously gathered patient data, it becomes possible to calculate an optimal insulin dose", explains Stephan Schaller. 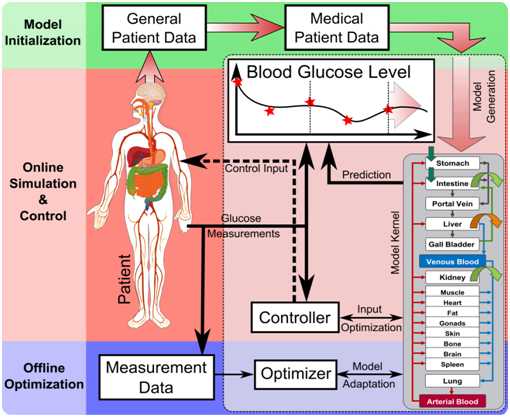 Figure 2 ©Bayer Technology Services: The workflow and information flow of an integrated system in a clinical environment during continuous closed-loop glucose control. The model kernel is initialised with general patient data (weight, height, gender). Blood glucose measurements are taken frequently, stored and the most recent measurements are delivered to the Controller. The process works within two timeframes: the online calculation of the optimal insulin dose (Control Input for closed-loop glucose control) based on recent glucose measurements and the offline “model adaptation” based on the full measurement data history in an extended timeframe.
Validation of the model
The algorithm for the integrated closed-loop control system was first validated in computer simulated clinical trials followed by a feasibility study involving 24-hour clinical trials on 10 patients with diabetes type1 at the Medical University of Graz in January and February 2013. The results show that glycaemic control in patients with type 1 diabetes can be achieved with the developed control approach using individualised Physiologically Based Pharmacokinetic/Pharmacodynamic model kernels within a robust Model Predictive Control framework and that large-scale computer simulated models of the glucose metabolism can provide a framework to improve diabetes research, the development of automatic control strategies for diabetes and ultimately everyday diabetes management. Future development of the physiological model includes improving automatic glucose control of patients with diabetes type 1 and modelling the pathological conditions of patients with diabetes type 2.
Related paper: A Generic Integrated Physiologically based Whole-body Model of the Glucose-Insulin-Glucagon Regulatory System in CPT: Pharmacometrics & Systems Pharmacology
/lbr/
to
the top  |  |
 |
 |
|
 |
 |
|
 |
|




 It is a software system for improving the workflow of clinical professionals (nurses, doctors) when managing patients with diabetes type 2 in general wards. In addition, the software supports clinical professionals in determining the right insulin dose for patients with diabetes type 2. Medical data (blood glucose, creatinine), physiological data (age, weight) and demographic data (age) entered manually by clinical professionals or received automatically from the hospital information system are used in accordance with the clinical protocol to calculate the insulin dose. Visualisation of BG values and medication is provided and the workflow is supported by automatically displaying outstanding tasks.
It is a software system for improving the workflow of clinical professionals (nurses, doctors) when managing patients with diabetes type 2 in general wards. In addition, the software supports clinical professionals in determining the right insulin dose for patients with diabetes type 2. Medical data (blood glucose, creatinine), physiological data (age, weight) and demographic data (age) entered manually by clinical professionals or received automatically from the hospital information system are used in accordance with the clinical protocol to calculate the insulin dose. Visualisation of BG values and medication is provided and the workflow is supported by automatically displaying outstanding tasks.



