To validate the REACTION platform in primary care, a series of field trials will be conducted at Chorleywood Health Centre (CHC), using existing clinical infrastructures.
With the expectation that the REACTION platform can greatly help reduce the risk of developing the long-term complications typically associated with diabetes and therefore the rate of hospital admissions, REACTION partners have developed manuals and deployment plans for the primary care setting, describing the procedures for the installation and configuration of the REACTION prototype.
Chorleywood Health Centre is a medium sized general practice with 6000 patients of which 145 are on the disease register for Diabetes – 27 Type 1, 118 Type 2 and 45 are currently on insulin therapy. REACTION will develop the platform to support the healthcare professionals in the management of these patients and to support the patient and carers of CHC.
The primary care prototype is based on a set of application specifications of which the general platform requirements include control and management of patients outside hospitals. The platform will facilitate therapy compliance and support for self-management, personalised feedback to clinicians and patients and medium-term risk assessment for patient education and lifestyle change support.
The aim of the primary care application is to provide clinicians with a tool for glycaemic control and risk assessment and to help patients manage their diabetes better.
Simultaneous monitoring
Emphasis is on the complex patient which means that the co-existence of other (chronic) illnesses must be taken into account in managing and treating diabetes. Therefore the REACTION project will aim to specify and validate a suite of services looking at simultaneous monitoring of blood glucose, blood pressure, physical activity, diet and co-morbidities to achieve comprehensive protection against diabetic complications and promote pro-active disease management.
The REACTION primary care application will also support medication compliance, adherence to clinical pathways, education and self-management health services for diabetes related conditions.
Primary care client, patient portal and clinical portal
The primary care prototype consists of three main components:
• The hub and devices which will be located in the patient’s home and used to collect
physiological data
• The patient portal, which will be used to collect activity, diet and other lifestyle data while also
providing a way for the clinician to give feedback to the patient
• The clinical user web interface, which will be used by health professionals to manage
patients, users and physiological data received from the
patient
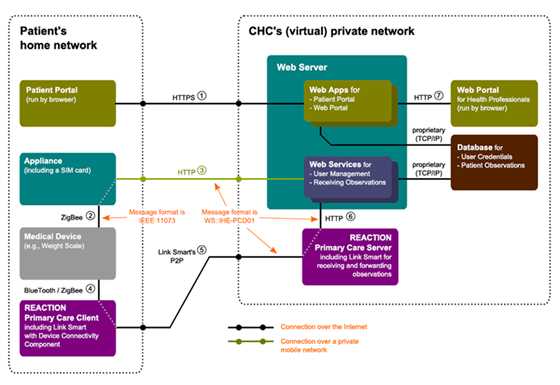
When the REACTION Primary Care Client application has been configured and deployed in the patient’s home, the patient can simply turn on the PC and start taking measurements from his devices e.g. for blood glucose, blood pressure and weight. The results will automatically be sent to the clinical staff via a gateway, plugged into a wall socket.
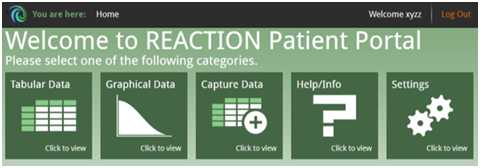
By using the Patient Portal it is possible for the patient to access data which have been submitted. The data are displayed either graphically or in tables.
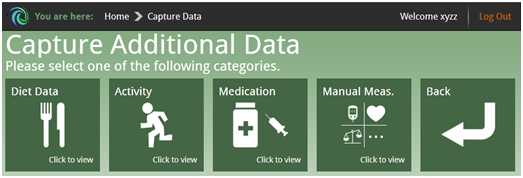
To support lifestyle management and support medication compliance, it is also possible for the patient to enter additional data on diet, physical activity, medication and other manual measurements and get general advice. The patient can submit the amount of insulin units and watch the history of dosage, thus keeping a medication diary.
Through the REACTION Clinical Portal, the clinician can view the readings from patients. Data are displayed by priority e.g. patients who have missed data or if data are outside of set parameters, the patient involved will feature at the top of the webpage, highlighted in red. The clinician can also view all expected readings of the day, a list of all patients and details of the individual patient such as risk level, educational content, monitoring data and disease category. It is also possible to specify monitoring thresholds for a patient and assign equipment and questionnaires to the patient.
The evaluation of the clinical field trial will be published in the public deliverable D8-4 Clinical evaluation of primary care clinical study which is due in August 2013. Partners involved in the development of the primary care prototype are FORTH-ICS, FORTHNET, UBRUN, CNET, CHC.
/lbr/
to
the top  |