A mobile glucose management system prototype has been designed by REACTION partners MUG, MSG and FORTH to support continuous glycaemic control of admitted patients with diabetes. The overall aim is to reduce the mortality and morbidity rate related to in-hospital hyperglycaemia.
Glycaemic control in the hospital of acutely ill patients with diabetes is often considered secondary in importance. However, studies demonstrate that in-hospital hyperglycaemia has been found to be an important marker of poor clinical outcome and mortality among diabetic patients and that intensive treatment of diabetes and hyperglycaemia shows positive results in terms of reduced mortality and morbidity. For this reason, patients
suffering from diabetes require continuous glycaemic control during hospital stays including close monitoring of blood glucose and determination of suitable treatment strategies.
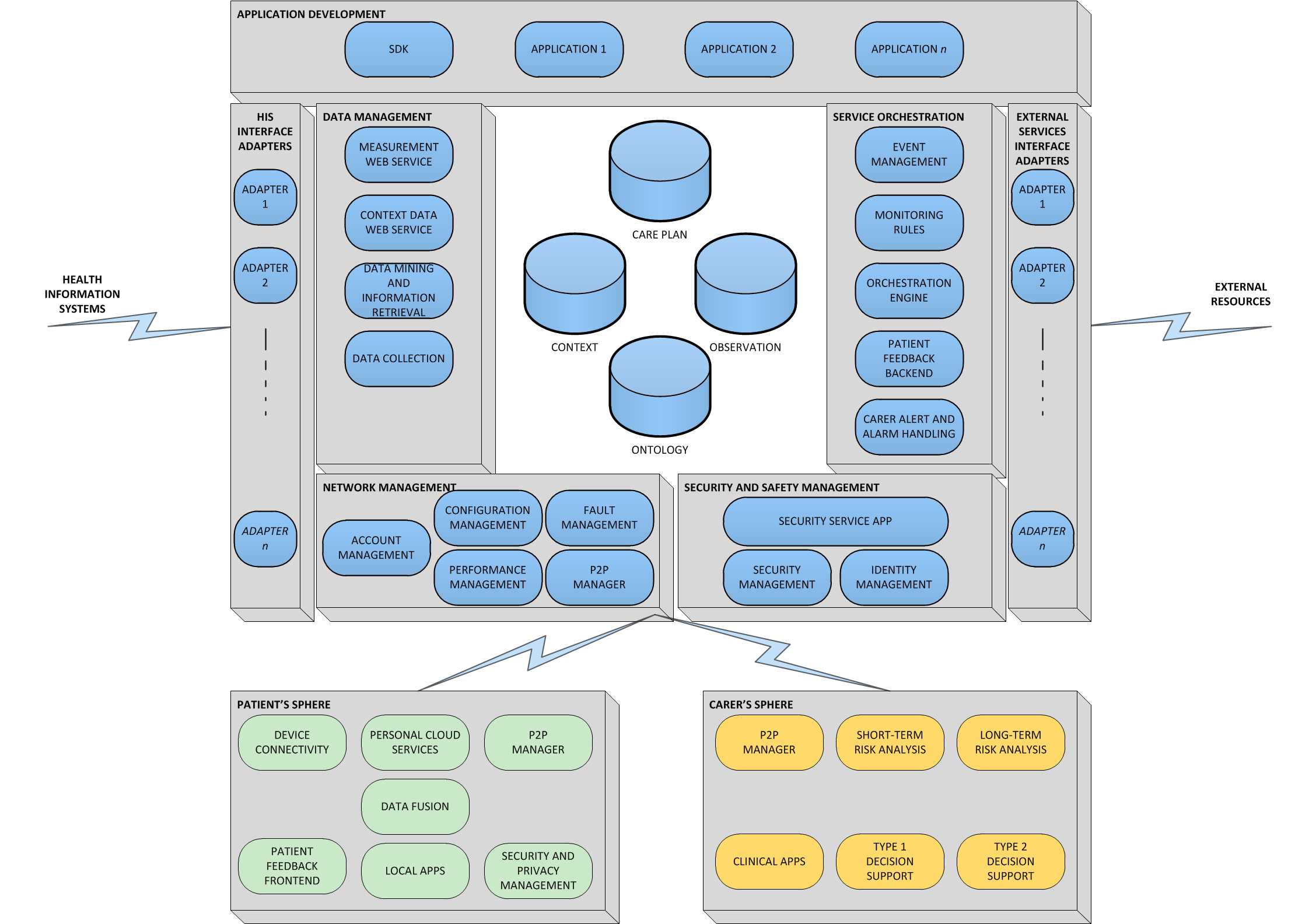
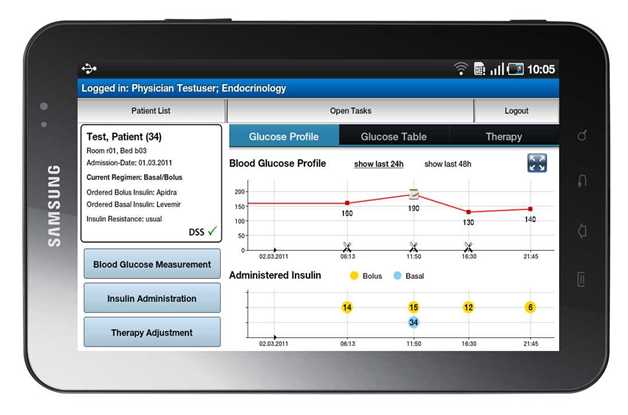 To support glycaemic control, REACTION partners MUG, MSG and FORTH have designed a first stage in-hospital glucose management system in close collaboration with clinical users. The
result is an android-based mobile client/server application which visualises the most important measurement and insulin administration parameters and advises on the needed insulin dosage of the patient. The system is accessed through a touch screen tablet PC for easy use for nurses and clinicians at patient beds.
To support glycaemic control, REACTION partners MUG, MSG and FORTH have designed a first stage in-hospital glucose management system in close collaboration with clinical users. The
result is an android-based mobile client/server application which visualises the most important measurement and insulin administration parameters and advises on the needed insulin dosage of the patient. The system is accessed through a touch screen tablet PC for easy use for nurses and clinicians at patient beds.
Measuring blood glucose, adjusting therapy and administering insulin
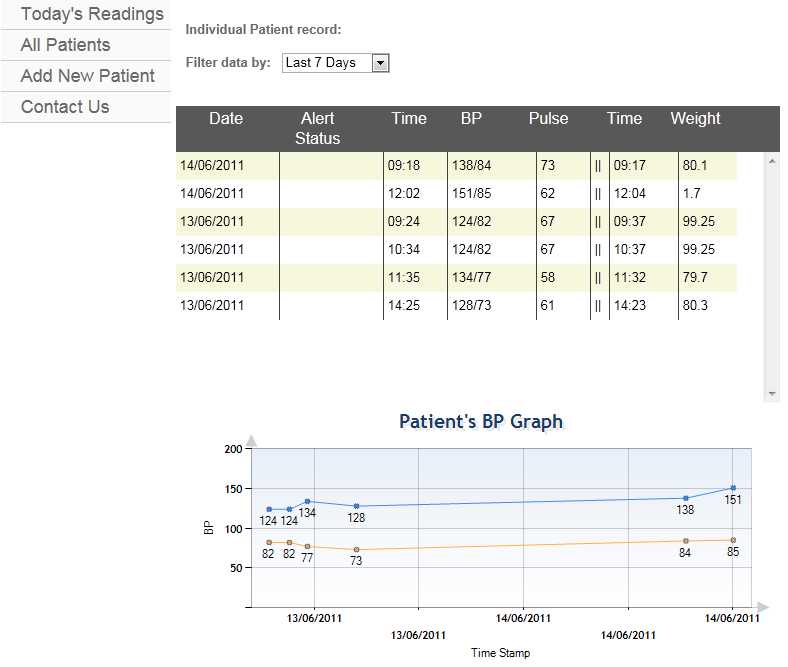
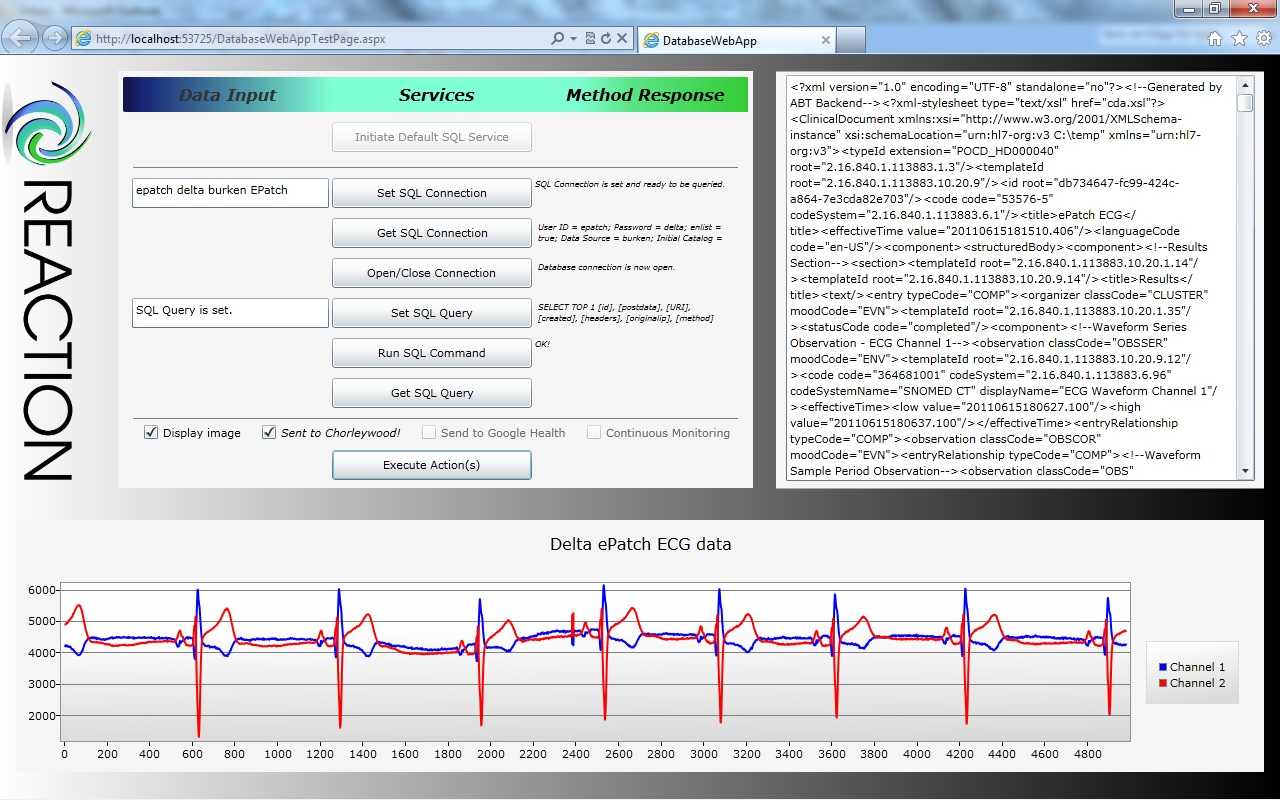
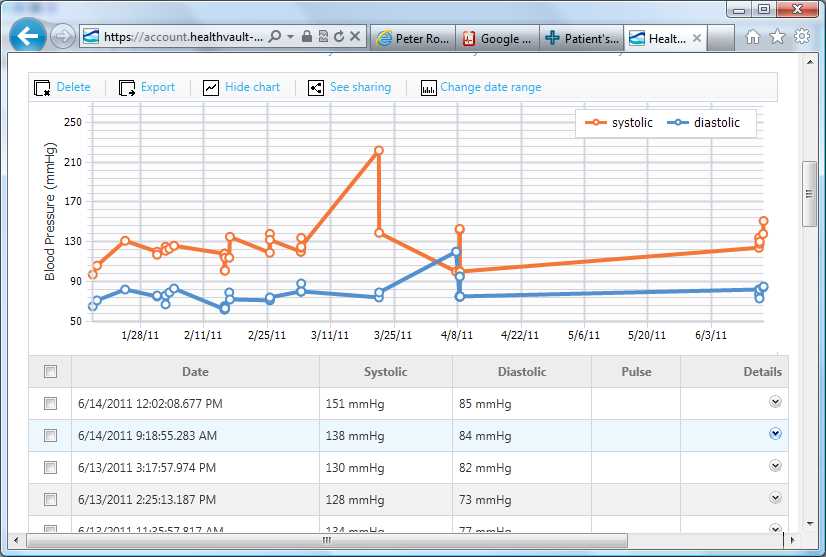
On a tablet PC the hospital staff can immediately access important patient data; where the patient is located, the date of admission to hospital and information about the current treatment and prescribed medication. Via the function Blood Glucose Measurement it is possible to retrieve the blood glucose value directly from the Laboratory Information System and document the measured values in the glucose management system.
Physicians approve the current therapy for the patient (e.g. insulin medication, current insulin dosage, hypoglycaemia borders) using the function Therapy Adjustment. Finally, in Insulin Administration, the decision support protocol for
insulin dosing suggests the needed insulin dosage of the patient based on the measured blood glucose values and food intake. No data is stored on the mobile unit, but on the backend server which also interfaces the Hospital Information System and ensures the calculations for decision support.
Different points of view concerning software
In designing the application for medical use, the challenge has been to consider a number of complex factors such as workflow requirements, usability, glucose control protocols and clinical safety. To understand the workflow and requirements, it was necessary to involve clinicians and the environment in which they work. For this purpose, a team of physicians, nurses and engineers from the Medical University of Graz, as well as engineers from the Institute of Medical Technologies and Health Management (MSG) at Joanneum Research, was established to develop the user interface design and the functionalities of the system. This proved to be a challenge since expectations to software differed considerably.
- The experiences through the iteration steps show that clinicians and engineers have very different points of view concerning software. While engineers often focus on gathering as much functionality as possible, clinicians prefer software which offers only the required base functionality but a sophisticated user interface, tailored to current workflow patterns, explains Stephan Spat, Researcher at MSG.
- A problem which has been encountered during the requirement analysis is that endusers often do not know what specific functions should be provided by a software solution. As a consequence, a paper mock-up which simulated the full system functionality on a mobile device was used as a trigger to give clinicians a preliminary idea as to how an in-hospital glucose management system, including a computerised decision support, could look, says Stephan Spat.
Through several steps of conceptualising, designing and testing, the results were integrated into an intuitive software system based on essential, but user-tailored functionalities.
The question of safety and privacy
One challenge is to understand what the physicians need, another is to safeguard the sensitive and personal information which is being processed. A key focus in REACTION is on security and data protection. Since tablet PCs will be connected wirelessly via computer networks (WLAN) or via mobile networks, misuse of data is possible. Therefore, to prevent confidentiality breaches, security measures have been taken such as incorporating data
encryption and digital signatures. In this respect, REACTION partner Fraunhofer SIT from The Fraunhofer Institute for Secure Information Technology is developing a security service application (SSA) for tablet PCs running Google’s Android Operating System.
- The SSA allows the glucose management system on the tablet to confidentially communicate with the hospital system, i.e. eavesdropping on the data transmitted is ruled out. It also supports mutual authentication between the tablet user and the hospital system to ensure that only authorised personnel can access or change the medical data of patients, explains Dr. Matthias Enzmann from Fraunhofer SIT.
For authorisation the SSA will include an identity management system that allows users to maintain and manage different identities or roles depending on the context at hand. In addition, strong accountability will be provided as an option.
- This means that sensitive actions of a health professional, e.g. an adjustment of a patient’s treatment or a change of therapy can be digitally signed, binding the signer’s identity to the requested action. This would be similar to the paper-based case today. Keeping such records in electronic form still allows for audits as electronically ordered actions can be traced back to the person who ordered them by way of her/his digital signature. In addition, it is easier to search for electronic records, they are accessible from any place (given proper authorisation) and require less space than paper-based records, says Dr. Matthias Enzmann.
Presently, the SSA allows for confidential communication and mutual authentication, though its identity management component is not yet complete. The support for strong accountability is not implemented yet and will be added later on in the project.
Validating the prototype
The next step will be to validate the protocol for insulin dosing on paper in a clinical trial at the Medical University of Graz. Based on the results, adaptations of the protocol will be integrated into the mobile software system. According to the medical device directive for software, all development steps will be documented and tested in detail. Finally, a second clinical trial will be conducted with a fully working mobile system for glycaemic control with decision support in the clinical environment of the Medical University of Graz.
In regard to the security aspect, the further configurement of SSA will entail assessing issues like ease of use and conformance to user expectation. The current prototype is based on monitoring patients with diabetes type 2 since it uses an algorithm (Rabbit 2) for diabetes type 2, however, plans are to use the system also for glucose management of patients with type 1 diabetes, by integrating an algorithm based on a model developed by partner BAYER.
/lbr/
to
the top  |



 To support glycaemic control, REACTION partners MUG, MSG and FORTH have designed a first stage in-hospital glucose management system in close collaboration with clinical users. The
result is an android-based mobile client/server application which visualises the most important measurement and insulin administration parameters and advises on the needed insulin dosage of the patient. The system is accessed through a touch screen tablet PC for easy use for nurses and clinicians at patient beds.
To support glycaemic control, REACTION partners MUG, MSG and FORTH have designed a first stage in-hospital glucose management system in close collaboration with clinical users. The
result is an android-based mobile client/server application which visualises the most important measurement and insulin administration parameters and advises on the needed insulin dosage of the patient. The system is accessed through a touch screen tablet PC for easy use for nurses and clinicians at patient beds.
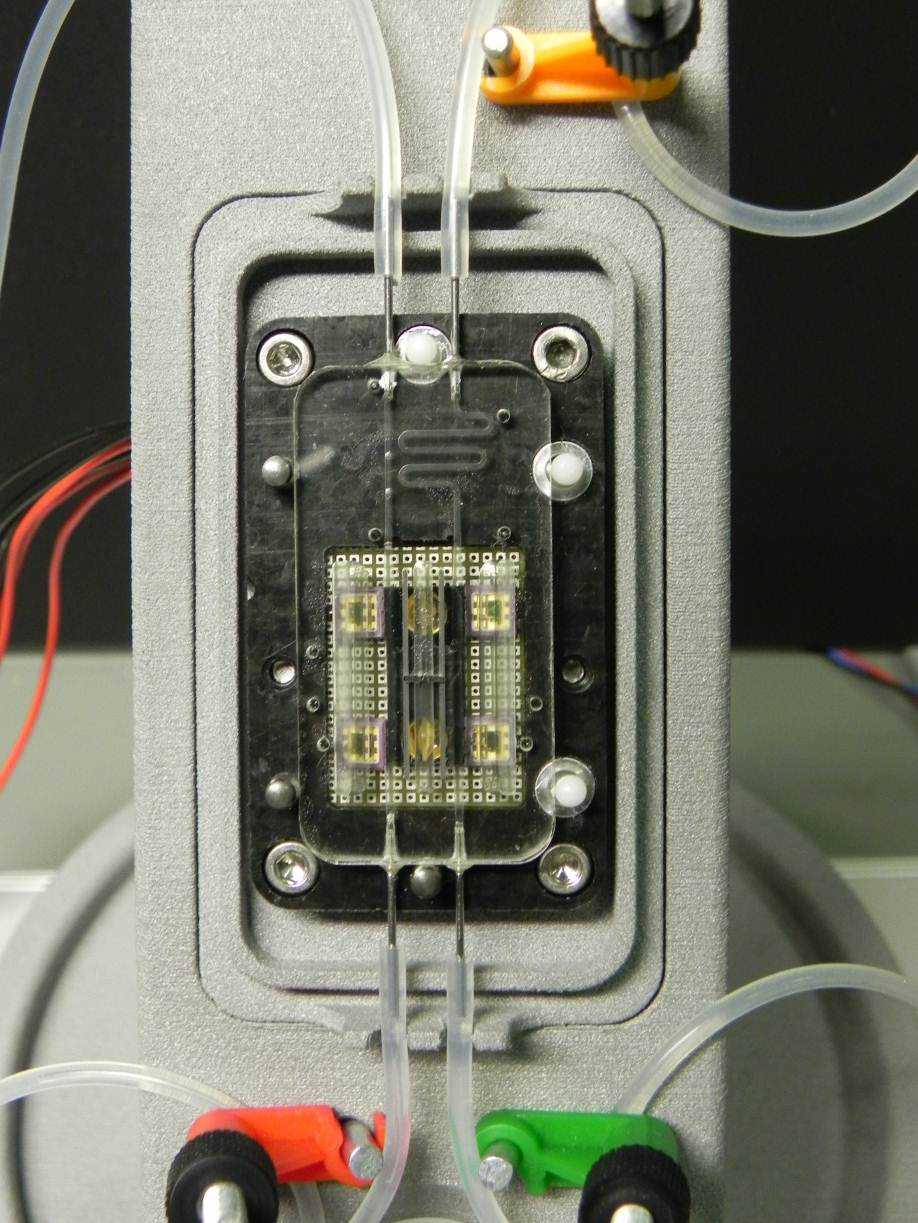
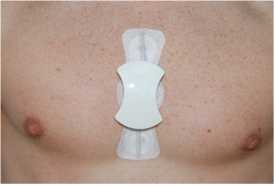
 One type of sensor is non-invasive with measuring technique based on impedance spectroscopy. Changes in glucose level cause changes in the skin’s impedance which is measured by electrodes in direct contact with the patient’s skin. The second type is minimally invasive with measuring technique based on infrared spectroscopy. Using a subcutaneous micro-needle, absorption spectroscopy is performed on the interstitial liquid or blood.
One type of sensor is non-invasive with measuring technique based on impedance spectroscopy. Changes in glucose level cause changes in the skin’s impedance which is measured by electrodes in direct contact with the patient’s skin. The second type is minimally invasive with measuring technique based on infrared spectroscopy. Using a subcutaneous micro-needle, absorption spectroscopy is performed on the interstitial liquid or blood.